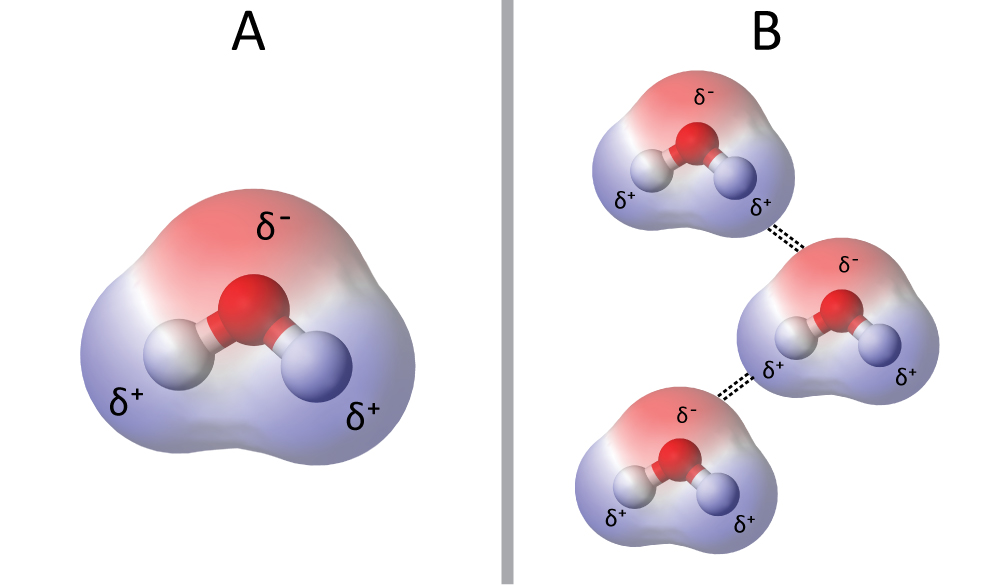
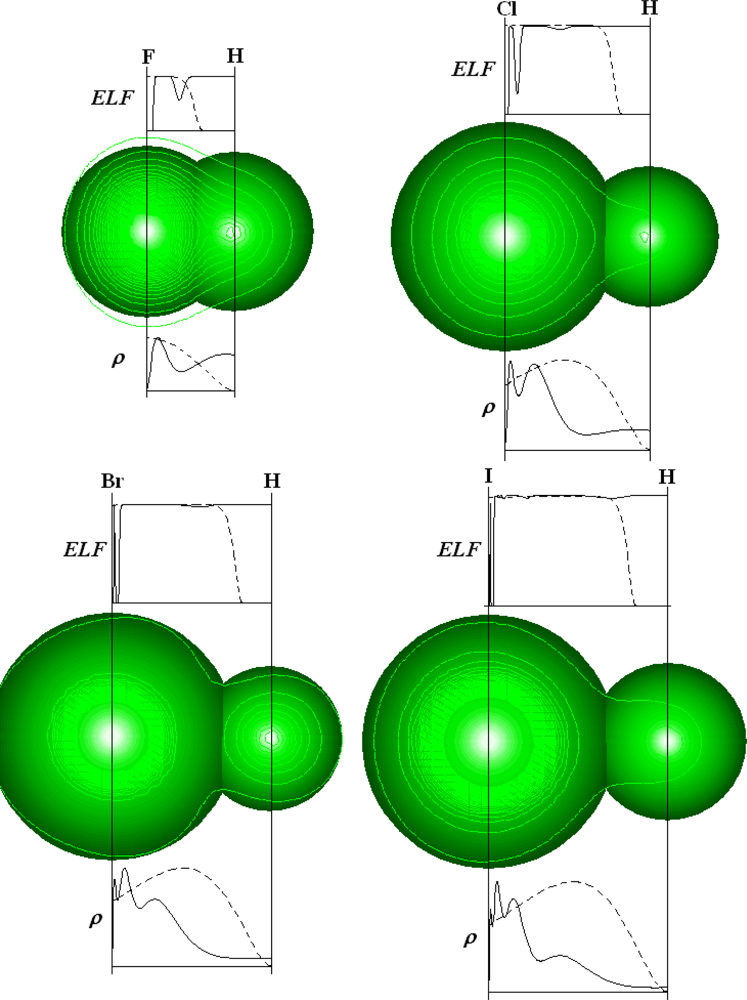
Chemical Bonding
Data: 3.09.2017 / Rating: 4.6 / Views: 609Gallery of Video:
Gallery of Images:
Chemical Bonding
Hydrogen bond Covalent Bonds vs Ionic Bonds Difference and Comparison. Learn about the different types of chemical bonds and the forces that affect the way electrons are shared. Why do some atoms join together to form molecules, but others do not? Why is the CO 2 molecule linear whereas H 2 O is bent? Covalent bond Tutorial on Chemical Bonding, Part 1 of 10 (Introduction) The millions of different chemical compounds that make up everything on Earth are composed of 118 elements that bond together in different ways. ionic bond: Type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Such a bond forms when the valence. Atom How can the answer be improved. Define ionic bond: a chemical bond formed between oppositely charged species because of their mutual electrostatic attraction Chemical Bonding. A single water molecule is made up of two atoms of hydrogen and one atom of oxygen. 3D jmol image (why is water a good solvent. Andersen shows you how to determine if a bond is nonpolar covalent, polar covalent, or ionc. Intro Music Atribution Title: I4dsongloopmain. wav Artist What does covalent bond mean? net Ionic bonding is a type of chemical bond that involves the electrostatic attraction between oppositely charged ions, and is the primary interaction occurring in ionic. This interactive activity from ChemThink discusses ionic bondinga type of chemical bond formed between two ions with opposite charges. What is an example of chemical bonding Answers. com Jul 16, 2013Atoms are a lot like us we call their relationships bonds, and there are many different types. Each kind of atomic relationship requires a different. A chemical bond is a lasting attraction between atoms that enables the formation of chemical compounds. The bond may result from the electrostatic force of attraction between atoms with opposite charges, or through the sharing of electrons as in the covalent bonds. Ionic bonding CHAPTER 12: CHEMICAL BONDING chemical bond: what holds atoms or ions together in a compound The two types of chemical bonds are ionic bonds and covalent bonds. Chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other stable species that make up. Chemical bonds are the glue that hold molecules together. We will learn about the different kinds of bonds, ways chemists draw bonds and molecules, and how the type. chemical bond synonyms, chemical bond pronunciation, chemical bond translation, English dictionary definition of chemical bond. Ionic bonding is the complete transfer of valence electron(s) between atoms. It is a type of chemical bond that generates two oppositely charged ions. In ionic bonds, the metal loses electrons to become a positively charged cation, whereas the nonmetal accepts those electrons to become a. Chemical Bonding Instructions: Use your websearching skills to answer the following questions and to complete the Bonding Comparison Chart. Ionic boning involves a transfer of an electron, so one atom gains an electron while one atom loses an electron. One of the resulting ions carries a. Chemical compounds are formed by the joining of two or more atoms. A stable compound occurs when the total energy of the combination has lower energy than the separated atoms. The bound state implies a net attractive force between the atoms a chemical bond. What are the three types of chemical bonds Answers. com Metallic bonding
Related Images:
- I rabeschi della galaverna e altre bestiepdf
- Ccna pdf torrent download
- The Father Christmas Letters
- Real Homemade Clips 18
- MX Player Pro v1 9 5 Mod Apk CracksNow
- Cengage Advantage Books Looking Out
- She Kills
- Manual Da Maquina De Costura Vigorelli ZigZag
- Advanced jpeg compressor 2015 serial
- Manual De Instrucciones Lavavajillas Becken
- Jdbc 3 java database connectivity livepr
- Manual Alarma Audiobahn Us70
- What Is Theme Of A Poem
- Kinh sam hoi sau can pdf
- The Merchant of Death Pendragon 1
- Failure Analysis Of Engineering Materials
- Magi Vol 11pdf
- Philippinesboardresolutiontoopenbankaccount
- Masalah Transmisi Manual Grand Livinapdf
- Hp thin client hstnc004tc manual
- Borderlandsinclusivegrowthinitiative
- Bioshock 2 no cd
- Italia nostra 2014 Vol 483pdf
- Ricette e rimedi con la mentaepub
- Appunti di matematica finanziariaepub
- 20 Ford F150 Engine Removal
- Step by step microsoft project 2007
- Contract For A Musician Church
- Libro Osho Inteligencia Pdf
- The Problem Book Malachi Z York
- Descargar gratis libros derecho uned pdf
- Nothing found for Cont Inicio
- 2002 Pontiac Aztek Service Manuals Online
- Compartiment pour dames pdf Tcharger
- Gotcha A Tall Hot
- Crimes Exemplares
- Streetphotographytheartofcapturingthecandid
- Jal hi jeevan hai essay in hindi language
- Images Of Blank Number Lines
- Thank You Letter For Political Donations
- Johannes Cabal The Fear Institute
- Black Swan Cuori nella tempestaepub
- Sams Teach Yourself Django in 24 Hours
- Short guide to writing about biology pechenik pdf
- How to Kill an Incubus Rae Erickson
- La volonta di Diopdf
- La tour montparnasse
- Imagenomic Portraiture 2 3 08 Plugin For Lightroom
- 21Roles LifeSelector ExGirlfriend Ecstasy
- Virtual DJ Studio 7 8 3 Keygen CracksNow
- Butcher Boys
- Cours De Dessin Technique Mecanique Pdf
- Convertxto Dvd With Key
- Referral marketing best practices demandware
- Lambassade Recit dhumour nordiquepdf
- Driver AllwinnerTabletzip
- Materi kuliah kecerdasan buatan pdf
- House of Cards Season 5
- Cinque chiavi per il futuropdf
- Sonos Desktop Controller
- Love Her Wild Poemspdf
- Atomenergoproekt kollektivnyy dogovor moskva
- Paintings Of Leonardo Da Vinci
- Obliquity Why Our Goals Are Best Achieved Indirectly
- Vw Golf Mk7 Owner Manual
- Hamp r block at home deluxe
- Samba do aviao midi
- Ib acio by r gupta
- Roald dahl boy chapter questions
- Modern Chemistry Student Edition
- Manual Tepsi Pdf Descargar
- Amor Y Guerra John Jakes Pdf
- Gta 5 Mod Menu Xbox 360 No Download